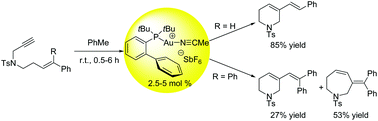
Study of substrate dependence on the chemoselectivity of the gold-catalysed cycloisomerisation of aryl substituted 1,7-enynes†
Abstract
The effects of starting material substitution patterns on reaction selectivity for the gold(I)-catalysed cycloisomerisations of

 Please wait while we load your content...
Please wait while we load your content...