Catalytic asymmetric synthesis of butane diacetal-protected (4S,5S)-dihydroxycyclohexen-1-one and use in natural product synthesis†
Abstract
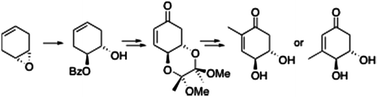
Due to the lack of availability of unnatural (+)-quinic acid as a starting material, a 6-step synthesis of butane diacetal-protected (4S,5S)-dihydroxycyclohexen-1-one (formally derived from (+)-quinic acid) has been devised. The key catalytic asymmetric step involves a chiral Co-salen-catalysed epoxide ring-opening reaction.

 Please wait while we load your content...
Please wait while we load your content...