Synthesis of the anti-influenza agent (−)-oseltamivir free base and (−)-methyl 3-epi-shikimate†
Abstract
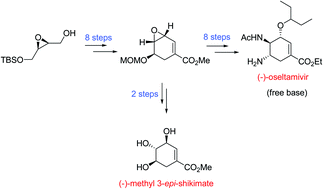
A new enantioselective synthesis of the anti-influenza agent (−)-oseltamivir free base (7.1% overall yield; 98% ee) and (−)-methyl 3-epi-shikimate (16% overall yield; 98% ee) has been described from readily available raw materials.

 Please wait while we load your content...
Please wait while we load your content...