Relaxation of the rigid backbone of an oligoamide-foldamer-based α-helix mimetic: identification of potent Bcl-xL inhibitors†
Abstract
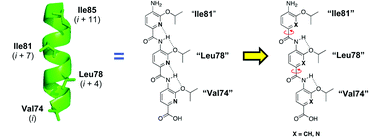
By conducting a structure–activity relationship study of the backbone of a series of oligoamide-foldamer-based α-helix mimetics of the Bak BH3 helix, we have identified especially potent inhibitors of Bcl-xL. The most potent compound has a Ki value of 94 nM in vitro, and single-digit micromolar IC50 values against the proliferation of several Bcl-xL-overexpressing cancer cell lines.

 Please wait while we load your content...
Please wait while we load your content...