Furanyl cyclic amines: a diastereoselective synthesis of 2,6-syn-disubstituted piperidines under thermodynamic control†‡
Abstract
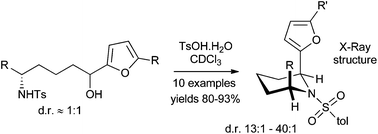
Utilising the propensity of the 2-furanyl group to facilitate equilibration of an adjacent tosylamide chiral centre, a diastereoselective route to 2,6-syn-piperidines was developed that proceeds with high levels of thermodynamic stereocontrol. X-ray crystallography structures suggest that, as seen in similar systems, pseudo-allylic strain between the N-tosyl group and the substituents at the 2 and 6 positions dominates stereochemical preference, overriding 1,3 diaxial interactions.

 Please wait while we load your content...
Please wait while we load your content...