Synthesis and photophysical evaluation of a pyridinium 4-amino-1,8-naphthalimide derivative that upon intercalation displays preference for AT-rich double-stranded DNA†
Abstract
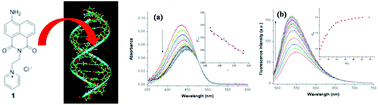
The synthesis, characterisation and solid state crystal structure of a cationic 4-amino-1,8-naphthalimide derivative (1) are described. The photophysical properties of 1 are shown to vary with the solvent polarity and H-bonding ability. The fluorescence of 1 is enhanced and blue-shifted in its 1 : 1 complex with 5′-adenosine-monophosphate while it is partially quenched and red-shifted in its complex with 5′-guanosine-monophosphate. Linear and circular dichroism measurements show that 1 binds to double-stranded DNA by intercalation. Comparative UV-visible and fluorescence studies with double stranded synthetic polynucleotides poly(dA–dT)2 and poly(dG–dC)2 show that 1 binds much more strongly to the AT polymer; 1 also has a strong preference for A–T rich sequences in natural DNA. Thermal denaturation measurements also reveal a much greater stabilisation of the double-stranded poly(dA–dT)2 than of natural DNA.

 Please wait while we load your content...
Please wait while we load your content...