A series of 7,12-dihydroindolo[3,2-d][1]benzazepine-6(5H)-ones (paullones) substituted at C9/C10 (Br) and C2 (Me, CF3, CO2Me) have been synthesized by a one-pot Suzuki–Miyaura cross-coupling of an o-aminoarylboronic acid and methyl 2-iodoindoleacetate followed by intramolecular amide formation. Other approaches to the paullone scaffold based on Pd-catalyzed C–H activation were unsuccessful. In vitro enzymatic assay with recombinant human SIRT-1 indicated a strong inhibitory profile for the series, in particular the analogue with a methoxycarbonyl group at C2 and a bromine at C9. These compounds are, in general, inducers of granulocyte differentiation of the U937 acute leukemia cell line and cause a marked increase in pre-G1 of the cell cycle.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
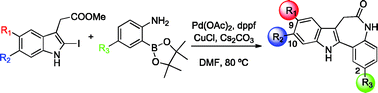

 Please wait while we load your content...
Please wait while we load your content...