Highly efficient asymmetric Michael addition of aldehyde to nitroolefin using perhydroindolic acid as a chiral organocatalyst†
Abstract
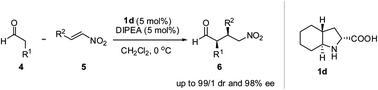
Perhydroindolic acids, the by-products obtained in the industrial production of a trandolapril intermediate, were used as chiral organocatalysts in asymmetric Michael addition reactions of
 Please wait while we load your content...
Please wait while we load your content...