Pyrrole versus quinoline formation in the palladium catalyzed reaction of 2-alkynyl-3-bromothiophenes and 2-alkynyl-3-bromofurans with anilines. A combined experimental and computational study†
Abstract
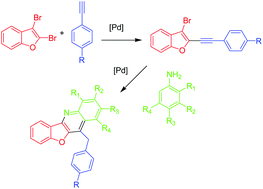
Benzofuroquinolines were prepared by a new type of Pd catalyzed annulation reaction. In the first step, 2-alkynyl-3-bromobenzofurans were prepared by Sonogashira reactions of 2,3-dibromobenzofuran. Their Pd catalyzed reaction with electron-rich anilines afforded benzofuroquinolines by a domino C–N coupling/annulation process. This reaction proceeds as a C,N-cyclization via the nitrogen atom and the ortho-carbon of the aniline. Similarly, furoquinolines were prepared from 2,3-dibromofuran. In contrast, benzofuropyrroles and furopyrroles were formed by N,N-cyclization when electron-poor anilines were used. Earlier, we reported results related to the thiophene and benzothiophene series. Quinolines were formed from 2,3-dibromobenzothiophene when electron rich anilines were used. In contrast, pyrroles were obtained in the case of electron-poor anilines. On the other hand, pyrroles were generally obtained, not depending on the type of aniline, when 2,3-dibromothiophene was employed as the starting material. In the present article, a detailed DFT study related to the mechanism (quinoline versus pyrrole formation) is reported which provides a rationalization of the selectivities observed for the furan, benzofuran, thiophene and benzothiophene series and for the different selectivities observed for electron-rich and -poor anilines.

 Please wait while we load your content...
Please wait while we load your content...