Protein supramolecular complex formation by site-specific avidin–biotin interactions†
Abstract
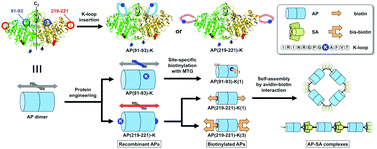
The precise accumulation of protein functions on a nanoscale to fabricate advanced biomaterials has become possible by a bottom-up approach based on molecular

 Please wait while we load your content...
Please wait while we load your content...