Stereoconvergent route to chiral cyclohexenone building blocks: formal synthesis of (−)-dysidiolide†
Abstract
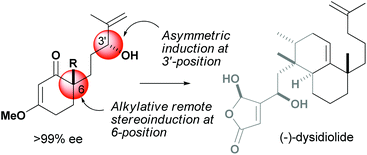
A stereoconvergent access to chiral carbocyclic building blocks is reported. 6-(3′-Hydroxy-4′-methylpent-4′-enyl)-3-methoxy cyclohex-2-enone (1) that consists of four stereoisomers, i.e., racemic ca. 1 : 1 diastereomers, is converted to enantiomerically pure carbocycles through a combination of regioselective catalytic asymmetric

 Please wait while we load your content...
Please wait while we load your content...