Triazole phosphohistidine analogues compatible with the Fmoc-strategy†
Abstract
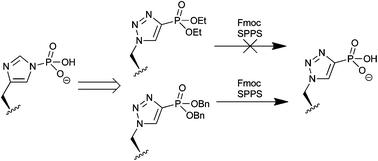
Phosphorylation of histidine is essential for bacterial two-component signalling; its importance to modulation of eukaryotic protein function remains undefined. Until recently, no immunochemical probes of this post-translational modification existed, however triazole phosphonate analogues of this modified amino acid have now been applied to the generation of site-specific antibodies. The protecting group strategy used in the original report is incompatible with standard protocols for Fmoc-solid phase peptide synthesis. In this paper, we report the application of P(III) chemistry to generate the complementary dibenzyl and di-tert-butyl phosphonate esters. These forms of the triazole analogue are fully compatible with standard Fmoc-SPPS and are therefore ideal for wider application by the chemical and biochemical community.

 Please wait while we load your content...
Please wait while we load your content...