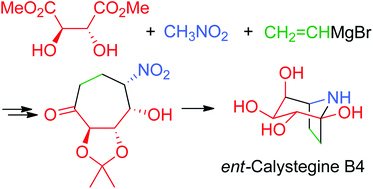
Total synthesis of ent-calystegine B4 via nitro-Michael/aldol reaction†
Abstract
Optically active ent-calystegine B4 was prepared in 13 steps from commercially available chiral L-dimethyl tartrate. The synthesis was achieved by the Michael addition and the aldol reaction of
 Please wait while we load your content...
Please wait while we load your content...