Diastereoselective synthesis of vicinal amino alcohols
Abstract
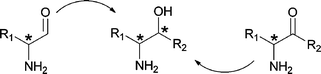
The vicinal amino alcohol is a common motif in natural products and pharmaceuticals. Amino acids constitute a natural, inexpensive, and enantiopure choice of starting material for the synthesis of such functionalities. However, the matters concerning diastereoselectivity are not obvious. This Perspective takes a look in the field of diastereoselective synthesis of vicinal amino alcohols starting from amino acids using various methods.
 Please wait while we load your content...
Please wait while we load your content...