Abstract
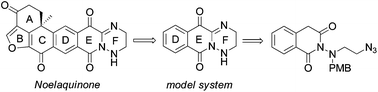
The intramolecular Staudinger-aza-Wittig reaction is used for a general synthesis of 1,2,5,6-tetrahydro-1,2,4-triazines, a structural motif reported for the natural product noelaquinone. The DEF moiety of noelaquinone was obtained in 13 steps and 2% overall yield, and the structure of the synthetic product was confirmed by
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...