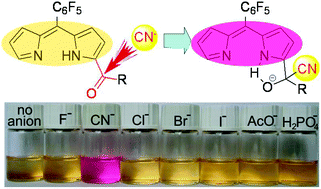
Cyanide sensing has attracted increasing interest due to its toxicity and wide use in industrial activities. Herein, we developed three colorimetric cyanide sensors by the modification of the α-position of a dipyrrin chromophore with various carbonyl groups, namely, C6F5CO, C6H5CO and CHO for 1, 2 and 3, respectively. In dichloromethane, these sensors respond to both CN− and F− with distinct colour changes. UV-Vis, 1H NMR and HRMS measurements imply a two-process interaction between the sensors and CN−. Initially, CN− forms a hydrogen bond with the NH moiety, and then it attacks the carbonyl group of the sensors via a nucleophilic addition reaction. In contrast, in aqueous systems, only cyanide induced vivid solution colour changes from light yellow to pink via nucleophilic addition reactions. The CN− detection limits reach a micromolar level of 3.6 × 10−6 M, 4.2 × 10−6 M and 7.1 × 10−6 M for 1, 2 and 3, respectively. In view of the easy synthesis and the highly selective recognition of CN− with vivid colour changes, 1–3 may be developed as a novel and promising prototype of selective and sensitive colorimetric cyanide sensors.
 Please wait while we load your content...
Please wait while we load your content...