Modulation of the antioxidant activity of phenols by non-covalent interactions
Abstract
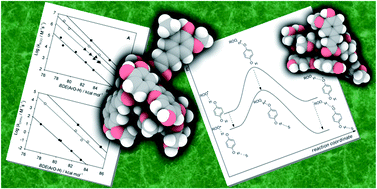
Non-covalent (H-bonding) interactions, either intramolecular or with the surrounding medium, have a major influence on the activity of natural and synthetic phenolic antioxidants, due to the modulation of their reactivity with radical species, such as peroxyl radicals. Different cases can be distinguished. (i) Intra- or inter-molecular H-bonding involving the reactive –OH moiety will depress the antioxidant activity if the –OH acts as H-bond donor, while the opposite will generally occur if it acts as H-bond acceptor. (ii) Remote intra- and inter-molecular H-bonding, involving a distant –OH group (in polyphenols) or a ring substituent, may increase or decrease the reactivity of an antioxidant toward free radicals, depending on whether the stabilization produced by the H-bond increases or decreases along the reaction coordinate, on proceeding from reactants to the transition state. In this Perspective, the role of non-covalent interactions in the complex chemistry of natural polyphenolic antioxidants is discussed with the aid of literature data on simplified model compounds, aiming at the composition of a clear picture that might guide future research.
 Please wait while we load your content...
Please wait while we load your content...