Development of a stereoselective Ugi reaction starting from an oxanorbornene β-amino acid derivative†
Abstract
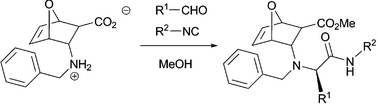
We have synthesised a novel oxanorbornene β-aminoacid derivative and employed it in a stereoselective Ugi reaction. Hypothesis regarding the mechanism taking place during the reaction have been made and validated through the determination of the relative and absolute configuration of the Ugi adducts. Use of the correct choice of solvents can increase stereoselection. The resulting bicyclic peptidomimetics can be used as a novel class of pluripotent substrates to be elaborated according to the synthetic strategies previously elaborated in our laboratories.

 Please wait while we load your content...
Please wait while we load your content...