SBA-15-functionalized palladium complex partially confined with ionic liquid: an efficient and reusable catalyst system for aqueous-phase Suzuki reaction†
Abstract
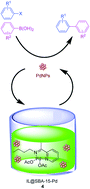
A novel SBA-15 functionalized palladium complex partially confined with 1-butyl-3-methylimidazolium hexafluorophosphate

 Please wait while we load your content...
Please wait while we load your content...