A new synthetic route for axially chiral secondary amines with binaphthyl backbone and their applications in asymmetric Michael reaction of aldehydes to nitroalkenes†‡
Abstract
A new synthetic route for binaphthyl-based
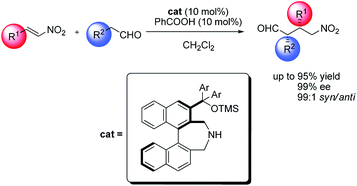
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...