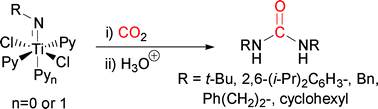
The coordinatively unsaturated 12-electron complex dichloro t-butylimido bispyridine titanium(IV) (2a) has been shown to react with CO2 to give N,N-bis-t-butyl urea. Two analogous sterically hindered coordinatively saturated 14-electron complexes dichloro t-butylimido trispyridine titanium(IV) (10a) and dichloro 2,6-(i-Pr)2phenylimido trispyridine titanium(IV) (10b) also gave their corresponding symmetrical ureas upon treatment with CO2. Further experiments support the intermediary of metallocycles formed from heterocumulene metathesis reactions. The unsymmetrical ureaN-benzyl, N-t-butyl urea (11) was produced from treatment of 2,6-(i-Pr)2phenylimido trispyridine titanium(IV) (10b) with CO2 and interception with BnNH2. Equimolar quantities of N,N-bistrimethylsilybenzylamine or N,N-bistrimethylsilyphenethylamine were shown to promote the reaction between t-butylimido bispyridine titanium(IV) (2a) and CO2 to give near quantitative yields of symmetrical urea. Other symmetrical ureas could be produced from TiCl4, amine and CO2 in moderate to quantitative yields depending on the stoichiometry of amine present.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...