Nanoionic transport and electrochemical reactions in resistively switching silicon dioxide
Abstract
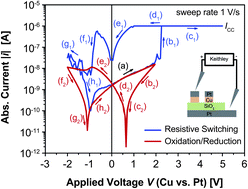
The mobility of copper ions and redox reactions of Cu at the interface with SiO2 being directly attributed to the resistive switching effect have been studied by

 Please wait while we load your content...
Please wait while we load your content...