One-step sonochemical synthesis of a graphene oxide–manganese oxide nanocomposite for catalytic glycolysis of poly(ethylene terephthalate)†
Abstract
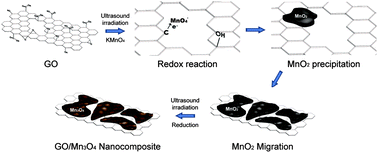
Ultrasound-assisted synthesis of a graphene oxide (GO)–manganese oxide nanocomposite (GO–Mn3O4) was conducted without further modification of GO or employing secondary materials. With the GO nanoplate as a support, potassium permanganate oxidizes the carbon atoms in the GO support and gets reduced to Mn3O4. An intensive ultrasound method could reduce the number of reaction steps and temperature, enhance the reaction rate and furthermore achieve a Mn3O4 phase. The composite was characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), scanning electron microscopy (SEM) and thermogravimetric analysis (TGA). The coverage and crystallinity of Mn3O4 were controlled by changing the ratio of permanganate to GO dispersion. The synthesized nanocomposite was used as a catalyst for poly(ethylene terephthalate) (PET) depolymerization into its monomer, bis(2-hydroxylethyl) terephthalate (BHET). The highest monomer yield of 96.4% was obtained with the nanocomposite containing the lowest amount of Mn3O4, while PET glycolysis with the Mn3O4 without GO yielded 82.7% BHET.

 Please wait while we load your content...
Please wait while we load your content...