Superatomic orbitals in sixteen-coordinate M@Li16 bonded by metallic bonds
Abstract
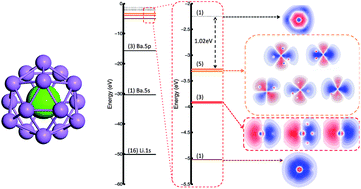
Based on density-functional calculation and genetic algorithm structure search, we propose a series of 16-coordinate core–shell clusters: M@Li16(M = Ca, Sr, Ba, Ti, Zr, Hf). A tetrahedral (Td) structure with an outer shell of 16 lithium atoms and one enclosed heavy atom is found to be the global minimum in the structural exploration of BaLi16 based on genetic algorithm. This structure also has lower energy compared to the other isomers we employed in all the MLi16 clusters. In this structure, the atoms are bonded together by metallic bonds with alkali (IA) and alkaline-earth (IIA) metal atoms. Their corresponding first electronic shells are closed with significant energy gaps because their total numbers of valence electrons fulfil the 18-electron rule. Such a combination could be extended to 20-electron systems by enclosing IVB elements. With simple valence electrons and highly symmetric structures, superatomic molecular orbitals are identified in all of the Td clusters.

 Please wait while we load your content...
Please wait while we load your content...