Synthesis of molecular gold clusters through a post-synthetic scheme involving HCl-promoted nuclearity convergence was examined with various phosphine ligands. Systematic studies with a series of bis(diphenylphosphino) ligands (Ph2P-(CH2)m-PPh2) using electrospray ionization mass spectrometry (ESI-MS) and electronic absorption spectroscopy demonstrated that the use of dppp (m = 3), dppb (m = 4) and dpppe (m = 5) as the ligands resulted in the formation of [Au13P8Cl4]+ type clusters, whereas the [Au13P10Cl2]3+ type cluster was formed with dppe (m = 2). The cluster species did not survive the HCl treatment step when monophosphines PPh3, PMe2Ph, and POct3 were employed, but [Au13(POct3)8Cl4]+ was isolated as a minor product in the NaBH4 reduction of Au(POct3)Cl in aqueous THF. Electronic absorption and photoluminescence studies of a series of Au13 clusters revealed that their optical properties are highly dependent on the phosphine/chloride composition ratio, but are far less so on the phosphine structure.
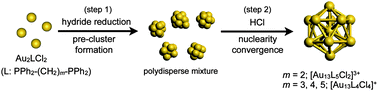
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
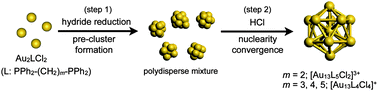

 Please wait while we load your content...
Please wait while we load your content...