Covering: up to the end of 2011
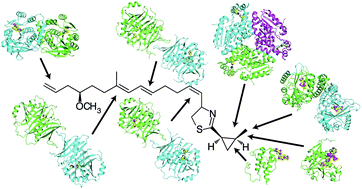
The world's oceans are a rich source of natural products with extremely interesting chemistry. Biosynthetic pathways have been worked out for a few, and the story is being enriched with crystal structures of interesting pathway enzymes. By far, the greatest number of structural insights from marine biosynthetic pathways has originated with studies of curacin A, a poster child for interesting marine chemistry with its cyclopropane and thiazoline rings, internal cis double bond, and terminal alkene. Using the curacin A pathway as a model, structural details are now available for a novel loading enzyme with remarkable dual decarboxylase and acetyltransferase activities, an Fe2+/α-ketoglutarate-dependent halogenase that dictates substrate binding order through conformational changes, a decarboxylase that establishes regiochemistry for cyclopropane formation, and a thioesterase with specificity for β-sulfated substrates that lead to terminal alkene offloading. The four curacin A pathway dehydratases reveal an intrinsic flexibility that may accommodate bulky or stiff polyketide intermediates. In the salinosporamide A pathway, active site volume determines the halide specificity of a halogenase that catalyzes for the synthesis of a halogenated building block. Structures of a number of putative polyketide cyclases may help in understanding reaction mechanisms and substrate specificities although their substrates are presently unknown.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...