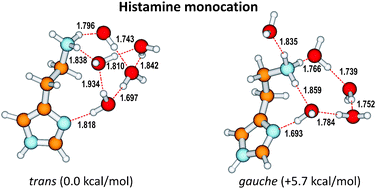
Computational methods were employed to investigate the nature of the microsolvation of the histamine monocation that corresponds to the aminoethyl protonated state of histamine under physiological conditions. The calculations were carried out at the M06-2X/6-311+G(d,p) and M06-2X/6-311++G(3df,3pd)//M06-2X/6-311+G(d,p) levels of theory in conjunction with the PCM solvent reaction field of Tomasi and co-workers. The results revealed that at least five explicit water molecules are required for the trans Nτ–H conformer to overcome the larger intrinsic stability of the gauche Nτ–H counterpart, the former being experimentally determined as the most dominant structure of histamine in water. The calculated N–H stretching frequencies, corresponding to the protonated aminoethyl group and to the ring amino moiety, together with the quantitative analysis of the strength of the solute–solvent hydrogen bonds, provide evidence that the –NH3+ fragment forms stronger hydrogen bonds with the neighboring solvent molecules than the ring NH group. This distinction could not be obtained by experiments alone. In this way computational results offer new insight and complement experiments on features highly relevant for the function of this biologically important molecule.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...