Complexes of silver(i) ions with tetrapyrrolidinyl- and tetramorpholinyl-PNP-lariat ethers in acetonitrile and methanol solutions
Abstract
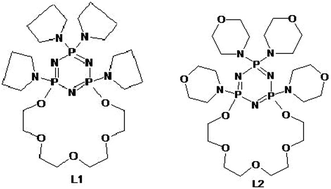
This work concerns the investigation of stability constants of silver(I) ions with the tetrapyrrolidinyl-PNP-lariat ether L1 in acetonitrile solution and tetramorpholinyl-PNP-lariat ether L2 in

 Please wait while we load your content...
Please wait while we load your content...