Fast and slow versus strong and weak metal–DNA binding: consequences for anti-cancer activity
Abstract
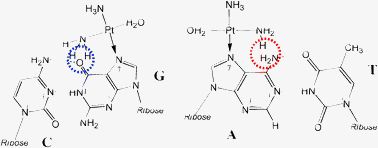
The binding of transition metal compounds to nucleic acids is discussed in the perspectives of kinetics and their anticancer activity. Kinetics of
- This article is part of the themed collection: Metals and Genetics

 Please wait while we load your content...
Please wait while we load your content...