Comparison between copper and cisplatin transport mediated by human copper transporter 1 (hCTR1)†
Abstract
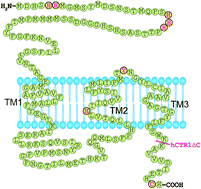
Copper transporter 1 (CTR1) is a transmembrane protein that imports copper(I) into yeast and mammalian cells. Surprisingly, the protein also mediates the uptake of platinum anticancer drugs, e.g. cisplatin and carboplatin. To study the effects of several metal-binding residues/motifs of hCTR1 on the transport of both Cu+ and cisplatin, we have constructed Hela cells that stably express a series of hCTR1 variant proteins including H22-24A, NHA, C189S, hCTR1ΔC, H139R and Y156A, and compared their abilities to regulate the accumulation and cytotoxicity of these metal compounds. Our results demonstrated that the cells expressing the hCTR1 mutants of histidine-rich motifs in the N-terminus (H22-24A, NHA) resulted in a higher basal copper level in the steady state compared to those expressing wild-type protein. However, the cellular accumulation of both copper and cisplatin in these variants was found at a similar level to that of wild type when incubated with an excess of metal compounds (100 μM). The cells expressing hCTR1 variants of H139R and Y156A exhibit lower capacities towards accumulation of copper but not cisplatin. Significantly, cells with the C189S variant partially retained the ability of the wild-type hCTR1 protein to accumulate both copper and cisplatin, while for cells expressing the C-terminus truncated variant of hCTR1 (hCTR1ΔC) this ability was absolutely abolished, suggesting that this motif is crucial for the function of the transporter.
- This article is part of the themed collection: Metals and Genetics

 Please wait while we load your content...
Please wait while we load your content...