Employing a polyketide synthase module and thioesterase in the semipreparative biocatalysis of diverse triketide pyrones†‡
Abstract
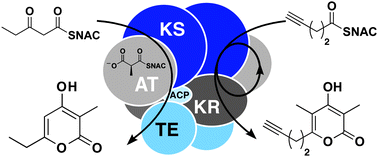
To demonstrate the potential of polyketide synthase (PKS) modules as biocatalysts that can synthesize polyketides in vitro, the terminal module and thioesterase (TE) from the erythromycin PKS were employed in the multimilligram, chemoenzymatic syntheses of diverse triketide pyrones. Methylmalonyl-S-NAC extender units were generated by the promiscuous malonyl-CoA ligase Streptomyces coelicolor MatB. Initiating the reaction with β-ketoacyl-S-NAC diketides yielded the anticipated triketide pyrones through chain extension and cyclization. TE-mediated hydrolysis of the substrates prevented quantitative yields; however, a reaction with 200 mg of diketide substrate generated 20 mg of pyrone product. Trace quantities of a particular pyrone were observed in all reactions and was hypothesized to result from extender unit decarboxylation and subsequent “stuttering”. To harness this polymerase-like activity, reactions were initiated with diverse acyl-S-NACs to yield the anticipated triketide pyrones, one harboring a terminal alkyne chemical handle. This biocatalytic system enables more informative analysis of in vitro PKS reactions by HPLC, NMR, and crystallography and sets the stage for the preparative generation of polyketides such as chiral building blocks valuable in the synthesis of natural products and pharmaceuticals.

 Please wait while we load your content...
Please wait while we load your content...