Thermo-resistant intrinsically disordered proteins are efficient 20S proteasome substrates†
Abstract
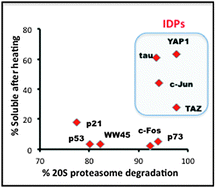
Based on software prediction, intrinsically disordered
- This article is part of the themed collection: Intrinsically Disordered Proteins

 Please wait while we load your content...
Please wait while we load your content...