Role of an intrinsically disordered conformation in AMPK-mediated phosphorylation of ULK1 and regulation of autophagy†
Abstract
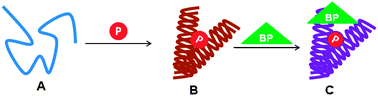
Recently, it has been established that there is a direct link between
- This article is part of the themed collection: Intrinsically Disordered Proteins

 Please wait while we load your content...
Please wait while we load your content...