Structure–property relationship of naphthalene based donor–π–acceptor organic dyes for dye-sensitized solar cells: remarkable improvement of open-circuit photovoltage†
Abstract
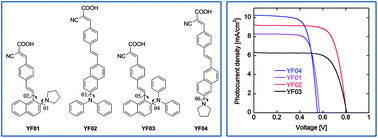
Four new donor–π–acceptor organic dyes (YF01–04), containing naphthalene-substituted amines as an electron donor and cyanoacrylic acid as an electron acceptor, were designed and synthesized, and their photophysical properties and dye-sensitized solar cells (DSCs) performances were characterized. Dyes YF02 and YF04, with 2,6-disubstituted naphthalene frameworks, were superior than their analog dyes YF03 and YF01, having 1,2-disubstituted naphthalene moiety, in incident-photo-to-current conversion efficiency (IPCE) and total solar-to-electric conversion efficiency (η). The DSCs based on YF02, comprised of diphenylamine moiety as the donor, produced the highest η of 5.29% compared to 4.03% of the analog dye YF04, which has pyrrolidine as the donor. Remarkably, a high open-circuit photovoltage (Voc) of 0.799–0.807 V was achieved in the cases of YF02–03, which have diphenylamine-donors. To better understand the structure–property relationship for DSCs application, molecular modelling was performed on YF01–04 and vertical electronic excitations were calculated using long-range corrected energy functional WB97XD and CAM-B3LYP at the basis set level DGDZVP, which were in excellent agreement with the experimental results. Moreover, the equilibrium molecular geometries of dyes YF01–04 were calculated at the density function theory (DFT) level using the hybrid energy functional B3LYP and basis set DGDZVP. The torsion angles (θ) between the naphthalene moiety and diphenylamine donor in YF02 and YF03 were more twisted than that of the pyrrolidine-donor dyes YF01 and YF04, precluding efficient intermolecular π–π charge transfer, which translated into high Voc. Compared to the reference dye TA-St-CA, which is based on diphenylamine as an electron donor linked to a phenyl ring, YF02 achieved higher Voc, which indicated that naphthalene substituted with diphenylamine is more efficient in retarding charge recombinations.

 Please wait while we load your content...
Please wait while we load your content...