A combined first principles and experimental study on Na3V2(PO4)2F3 for rechargeable Na batteries†
Abstract
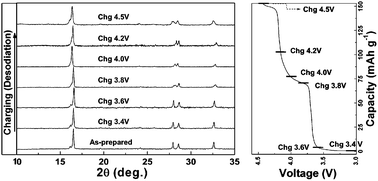
The electrochemical properties of Na3V2(PO4)2F3 in a Na rechargeable battery were investigated through a combined computational and experimental study. Ex situ

 Please wait while we load your content...
Please wait while we load your content...