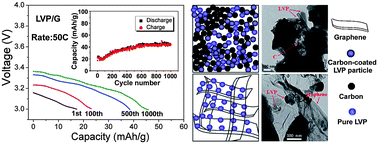
Recently, improvement on cycling stability and rate performance were reported when the electrode materials were supported by graphene. In this work, we report the approaches for fabricating a nano-structure Li3V2(PO4)3/carbon with conventional carbon-coating and Li3V2(PO4)3/graphene with graphene sheets supporting the composite. The crystal structure and morphology, the lithium diffusion behavior and high rates capacities of pure LVP, composites of LVP with conventional carbon and graphene sheets are studied in detail. The conventional carbon or some LVP particles are separately aggregated without effectively compounding with each other, but there is a more efficient carbon coating by graphene because the LVP nanoparticles are grown on or are enwrapped into a 2D network of graphene layers. Minor graphene contained in the Li3V2(PO4)3/graphene nanocomposite can result in a reduction of crystal size, a large surface area, an increase in conductivity (three orders of magnitude), and great improvement in the rate performance and cycling stability. We proposed an effective carbon coating (ECC) model of microstructure of LVP nanoparticles compounded with carbon or graphene to discuss the key roles of graphene on the great improvement of electrochemical performance. It should offer a new idea in the design and synthesis of battery electrodes based on carbon-coated technology.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...