Metathesis of alkali-metal amidoborane and FeCl3 in THF†
Abstract
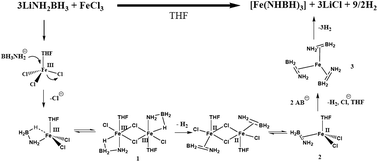
Metathesis of LiNH2BH3 and FeCl3 in THF solution was investigated in detail. Instead of formation of expected Fe amidoborane i.e., 3LiNH2BH3 + FeCl3 → 3LiCl + Fe(NH2BH3)3, 1.5 equiv. H2/LiNH2BH3 together with LiCl and a black precipitate was produced as a result of salt metathesis and reduction of Fe3+ by BH3. The hydrogen was desorbed in two steps involving a homogeneous interaction of the two starting chemicals to form [Fe(H2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2)3] precipitate and subsequent solid-state dissociation of [Fe(H2N
BH2)3] precipitate and subsequent solid-state dissociation of [Fe(H2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2)3] to yield a polymeric product, [Fe(HN
BH2)3] to yield a polymeric product, [Fe(HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH)3]n, respectively. FTIR evidenced the persistence of B–H and N–H stretches in the above two solid products and following the dissociation of [Fe(H2N
BH)3]n, respectively. FTIR evidenced the persistence of B–H and N–H stretches in the above two solid products and following the dissociation of [Fe(H2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2)3] to release 1 equiv. H2/LiNH2BH3 the B–N bond strengthened. Mössbauer and XAFS both indicated that Fe atoms in these solids are in very similar chemical environments, linking to the neighbouring N and B atoms and bearing slightly positive charge. Most likely, H2N
BH2)3] to release 1 equiv. H2/LiNH2BH3 the B–N bond strengthened. Mössbauer and XAFS both indicated that Fe atoms in these solids are in very similar chemical environments, linking to the neighbouring N and B atoms and bearing slightly positive charge. Most likely, H2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2 in [Fe(H2N
BH2 in [Fe(H2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2)3] binds to Fe as a π-bound ligand. The mechanism of salt metathesis and reduction of Fe3+ was confirmed based on simulation work on the homogeneous reaction process.
BH2)3] binds to Fe as a π-bound ligand. The mechanism of salt metathesis and reduction of Fe3+ was confirmed based on simulation work on the homogeneous reaction process.

 Please wait while we load your content...
Please wait while we load your content...