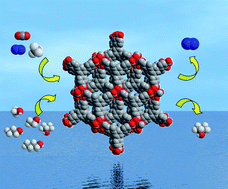
A novel homochiral ultramicroporous lanthanide–organic framework, Ce(BTB)(H2O) (1) (H3BTB = 1,3,5-benzenetrisbenzoic acid), with high surface area and two types of open ultramicropores has been synthesized under solvothermal condition, which exhibits an unusual stepwise hysteretic adsorption of O2 and N2 at 77 K and high-efficiency gas separations of CO2/N2 and CH4/N2 at 273 K. The ultramicropores of 1 lead to an unprecedented separation of the propanol isomers due to the slight differences of their geometry and dipole moments. Furthermore, the method for calculating the surface-area and gas separation for 1 is summarized. These will provide a general methodology that can be employed to simulate the surface area and gas separation properties of ultramicroporous materials.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...