Nanocrystalline hybrid inorganic–organic one-dimensional chain systems tailored with 2- and 3-phenyl ring monocarboxylic acids†
Abstract
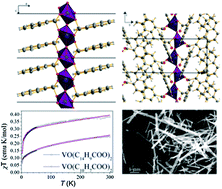
Two novel nanosized hybrid inorganic–organic frameworks, VO(C14H9COO)2, and VO(C10H7COO)2 have been solvothermally synthesized and their structures elucidated using a combination of
- This article is part of the themed collection: Integrating functionality into metal–organic frameworks

 Please wait while we load your content...
Please wait while we load your content...