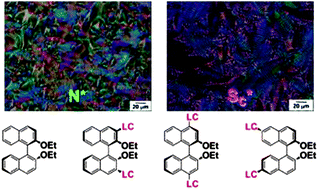
The chirality transfer of axially chiral binaphthyl derivatives bearing liquid crystal (LC) moieties at the n,n′ positions (n = 3, 4, 6) of the binaphthyl rings to nematic (N) and smectic (S) LCs is investigated. Chiral nematic LCs (N*-LCs) are prepared by adding a small amount of the chiral binaphthyl derivative into host N-LCs composed of cyanobiphenyl mesogen cores. The binaphthyl derivative with phenylcyclohexyl (PCH) type LC moieties at the 4,4′ positions of the binaphthyl ring [D-4,4′] exhibits a low helical twisting power (HTP) of 11 μm−1. In contrast, those with LC moieties at the 3,3′ and 6,6′ positions of the binaphthyl rings [D-3,3′ and D-6,6′] exhibit high HTPs of 153 μm−1 and 154 μm−1, respectively. Next, the binaphthyl derivatives are added into two types of S-LCs with phenylbenzoate mesogen cores: 4-(4-methylpentyloxy)phenyl-4-(decyloxy)benzoate [PhB1] and 4-(3-methylpentyloxy)phenyl-4-(decyloxy)benzoate [PhB2]. The mixture of the host LC, PhB1 or PhB2 with the chiral dopant, D-3,3′ or D-6,6′ shows chiral smectic LCs C (SC*-LCs). The highly twisted SC* phases with helical pitches of 1.2–1.4 μm are prepared in PhB1 and PhB2 by using the chiral dopant of D-6,6′. It is concluded that D-6,6′ has a large helical twisting power and is the most favourable atropisomeric chiral inducer for chirality transfer to both N-LCs and S-LCs.

 Please wait while we load your content...
Please wait while we load your content...