Kinetics and pathways for an algal phospholipid (1,2-dioleoyl-sn-glycero-3-phosphocholine) in high-temperature (175–350 °C) water
Abstract
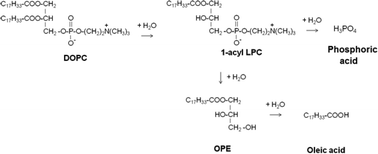
We examined the behavior of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) in high-temperature water at 175, 200, 225, and 350 °C. DOPC hydrolyzed to give oleic acid and a number of phosphorus-containing products. The hydrolysis was catalyzed by oleic and phosphoric acids, which were also reaction products. DOPC formed 1-acyl and 2-acyl lyso-phosphatidylcholine (LPC) along with oleic acid as primary products. LPC subsequently formed other phosphorus-containing intermediates, which finally led to phosphoric acid as the ultimate P-containing product. At 350 °C, phosphoric acid and oleic acid were the only products observed. We observed an ester of oleic acid and glycerol (9-octadecenoic-2,3-dihydroxypropyl ester), which likely formed via the hydrolysis of LPC. A reaction network is proposed to explain the formation of the observed products. A quantitative kinetics model based on the proposed pathways was consistent with the experimental data.

 Please wait while we load your content...
Please wait while we load your content...