“All-water” chemistry of tandem N-alkylation–reduction–condensation for synthesis of N-arylmethyl-2-substituted benzimidazoles†
Abstract
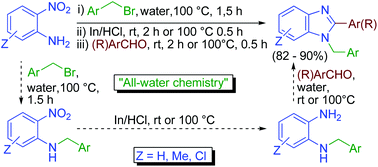
A water-assisted tandem N-alkylation–reduction–condensation process has been devised as a new synthetic route for the one-pot synthesis of N-arylmethyl-2-substituted

 Please wait while we load your content...
Please wait while we load your content...