Intramolecular etherification of five-membered cyclic carbonates bearing hydroxyalkyl groups†
Abstract
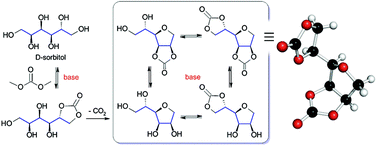
We report a new one-pot synthetic route to tetrahydrofuran derivatives, which were unexpectedly produced under basic conditions by intramolecular etherification of substituted five-membered cyclic

 Please wait while we load your content...
Please wait while we load your content...