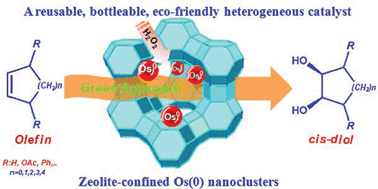
Addressed herein is a novel, eco-friendly, recoverable, reusable and bottleable catalytic system developed for the dihydroxylation of various olefins yielding 1,2-cis-diols. In our protocol, zeolite-confined osmium(0) nanoclusters (zeolite-Os0) are used as reusable catalyst and H2O2 served as a co-oxidant. Zeolite-Os0 are found to be highly efficient and selective catalysts for the dihydroxylation of a wide range olefins in an aqueous acetone mixture at room temperature. In all of the olefins surveyed, the catalytic dihydroxylation reaction proceeds smoothly and the corresponding 1,2-cis-diols are obtained in excellent chemical yield under the optimized conditions. The present heterogeneous catalyst system provides many advantages, such as being eco-friendly and industrially applicable over the traditional homogenous OsO4–NMO system for the dihydroxylation of olefins.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...