The use of environmental metrics to evaluate green chemistry improvements to the synthesis of (S,S)-reboxetine succinate
Abstract
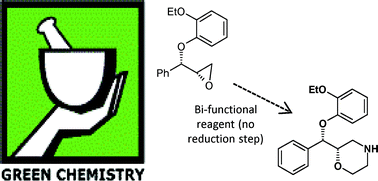
The Pfizer Green Chemistry metrics program is described and exemplified with a case history involving the synthesis of (S,S)-reboxetine succinate. The initial route used a classical resolution approach and generated high levels of waste. This route was replaced by an enantiospecific synthesis which used

 Please wait while we load your content...
Please wait while we load your content...