Copper molybdenum sulfide: a new efficient electrocatalyst for hydrogen production from water†
Abstract
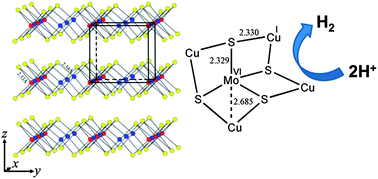
A new inorganic solid state electrocatalyst for the hydrogen evolution reaction (HER) is reported. Highly crystalline layered ternary sulfide copper-molybdenum-sulfide (Cu2MoS4) was prepared by a simple precipitation method from CuI and [MoS4]2− precursors. In aqueous solution and over a wide pH range (pH 0 to 7), this Cu2MoS4 showed very good catalytic activity for HER with an overvoltage requirement of only ca. 135 mV and an apparent exchange current density of 0.040 mA cm−2 (Tafel slope of ca. 95 mV per decade was found irrespective of the pH value). This Cu2MoS4

 Please wait while we load your content...
Please wait while we load your content...