Water-soluble, redox-active organometallic calcium chelators†
Abstract
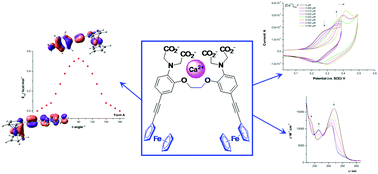
This paper describes a new series of organometallic water-soluble chelators combining a redox moiety (ferrocene) and a selective Ca2+ chelator (BAPTA) separated by an ethynyl bridge. We report the synthesis and characterization of organometallic derivatives of the BAPTA chelator featuring one (2a) and two ferrocenyl (2b) moieties. Single crystal X-ray structural analysis on these chelators revealed unexpected conformations for the ferrocenyl substituent with respect to the phenyl ring of the BAPTA unit. DFT calculations on a model system of the ferrocenyl-ethynyl-BAPTA molecule were carried out to evaluate the energy separation between the two limiting conformations observed experimentally in the solid state, and to check the effective electronic communication between the binding pocket and the redox probe. The binding affinity of 2a–b for Ca2+, as probed by UV-Vis and cyclic voltammetry, revealed distinct behaviors in the presence of a metal ion depending on whether BAPTA is substituted by one or two ferrocenyl groups.

 Please wait while we load your content...
Please wait while we load your content...