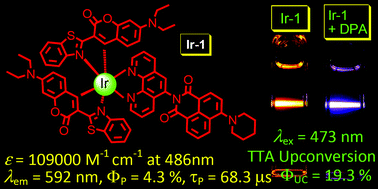
Visible light-harvesting cyclometalated Ir(III) complexes with 3-(2-benzothiazoly)-7-diethylaminocoumarin as the C^N cyclometalation ligands were prepared. The ancillary N^N ligand is either 6-piperidine naphthalimide-phenanthroline (Ir-1) or 9-aminophenanthroline (Ir-3). Ir(ppy)2(Phen) was prepared as model complex (Ir-2). Ir-1 and Ir-3 show strong absorption of visible light (ε = 109 000 M−1 cm−1 or 112 000 M−1 cm−1 at 486 or 484 nm, respectively). All the complexes show room temperature phosphorescence with drastically different phosphorescence quantum yields (ΦP = 4.3%, 44.3% and 46.0% for Ir-1, Ir-2 and Ir-3, respectively). With steady state and time-resolved spectra, as well as DFT calculations, the T1 excited states of Ir-1 and Ir-3 were proposed to be the 3IL state, whereas the 3MLCT state was proposed for Ir-2. Long-lived emissive triplet excited states (7.6 μs and 54.5 μs) were observed for Ir-1 and Ir-3, compared to the short T1 excited state lifetime of Ir-2 (1.2 μs). The complexes were used as triplet photosensitizers for triplet–triplet annihilation upconversion and upconversion quantum yields (ΦUC) of 19.3% and 12.7% were observed for Ir-1 and Ir-3, respectively. No upconversion was observed for Ir-2 under the same experimental conditions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...