The synthesis and characterisation of novel ferrocenyl polyphenylenes†
Abstract
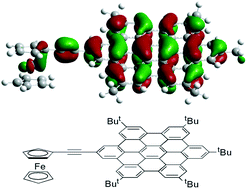
This work describes the synthesis and characterisation of a new series of polyphenylenes with up to four ferrocenyl moieties. The synthetic route involves the preparation of a number of novel precursors. Cyclopentadienones, generated from the two-fold Knoevenagel condensation of di-ferrocenyl propanones and

 Please wait while we load your content...
Please wait while we load your content...