Chemistry of the heavy group 15 elements with the pyridyl tethered 1,2-bis(imino)acenaphthene “clamshell” ligand†
Abstract
Cobaltocene has been used as a one-electron reductant in a facile route to generate pnictogen(I) (P, As) synthons. These subsequently undergo a formal 4 + 2
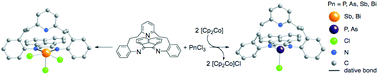
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...